How Thalidomide Went From Medical Disaster to Miraculous Cancer Treatment
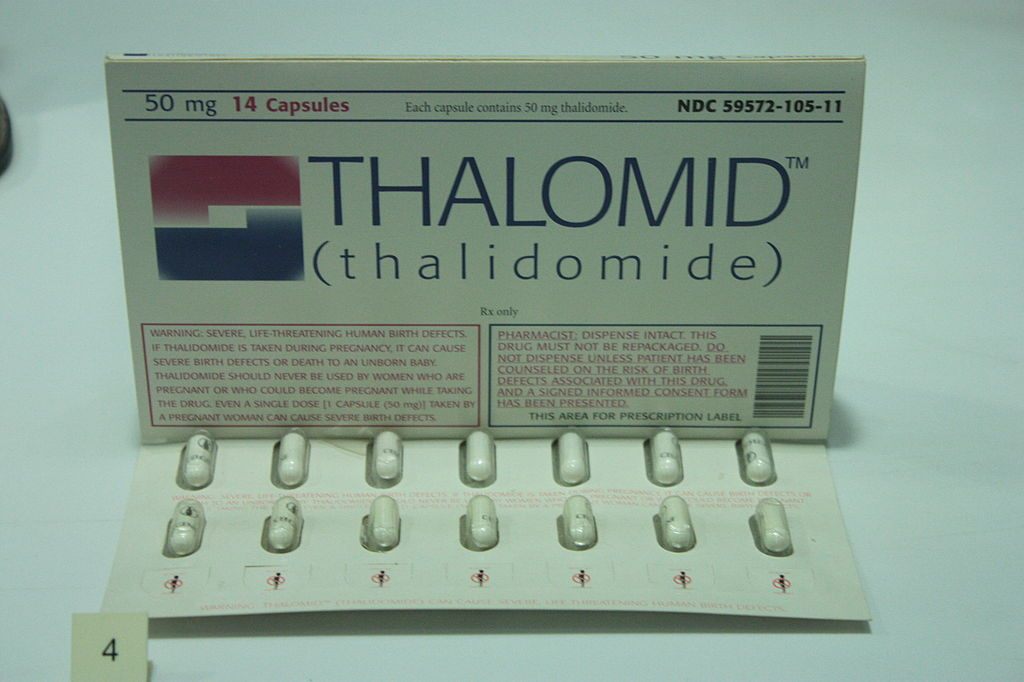
A packet of thalidomide tablets, c. 1960, on display at the Science Museum in London. (Photo: Stephencdickson/WikiCommons CC BY-SA 4.0)
In 2012, a crowd gathered to see the unveiling of a bronze sculpture in Stolberg, Germany. The artwork depicted a girl, reaching toward the sky–but her hands began at the ends of her shoulders; she lacked arms. Her feet were deformed, her mouth open in a silent scream. Next to her, another bronze chair sat empty. The president of Grünenthal, the German company who commissioned the statue, began a speech: “We have a responsibility and we face it openly,” he said. “We’ve been silent, and we’re very sorry for that.”
This event was part of an apology by Grünenthal for their drug thalidomide, more than 50 years after it caused what is often considered the world’s most tragic medical catastrophe, when scores of children whose mothers had taken it during pregnancy were born with blindness, brain damage, and malformed limbs.
Thalidomide’s legacy goes far beyond disaster, though. Despite ushering in much medical terror in its heyday, the drug has resurfaced in unexpected ways in recent years–including as a miracle drug for treating cancer.
In the late 1950s, Grünenthal (then called Chemie Grünenthal) developed thalidomide as a sedative, released in West Germany as Contergan, and under various other names across the world. It was the new wonder drug: it also reduced nausea, and best of all was safe, with studies indicating a person could eat it in unlimited quantities. Doctors, elated, received billions of pills and prescribed them to hundreds of pregnant women for morning sickness and insomnia.
But by the early 1960s, doctors around the world noticed alarming birth trends: 40 percent of children born to mothers who took thalidomide during pregnancy died, and survivors suffered from side effects like phocomelia, the shortening or absence of limbs. Thousands of thalidomide victims, known as “thalidomide babies,” were born in at least 45 countries around the world.

Frances Kathleen Oldham Kelsey receiving the President’s Award for Distinguished Federal Civilian Service from President John F. Kennedy, in 1962. (Photo: Public Domain/WikiCommons)
The United States largely dodged the thalidomide tragedy, with just 40 victims reported here, thanks to FDA medical officer Dr. Frances Kelsey, who barred the drug’s release after she discovered that a company representative–not an independent lab–had published the drug’s safety studies and advocated its rampant use. For the rest of the world, this medicine was recalled almost universally by 1962, once the word spread.
In the aftermath, some countries still had small amounts of the medicine hanging around. Those remaining pills would change the way scientists thought about treating disease, up to the present day.
In 1964, Israeli doctor Jacob Sheskin had patients with harsh leprosy symptoms involving painful lesions. While looking for any possible drug available, he came upon thalidomide in his office’s apothecary. He gave it to the patient, and it worked almost immediately: the lesions practically disappeared overnight.
Doctors began using it for other illnesses that seemed biochemically related, and found another astonishing effect: it elongated the lives of people with bone and blood cancers. In 1999, Dr. Bart Barlogie of the University of Arizona studied 84 patients who took the drug: a third of the patients improved while two went completely into remission. More studies about how this worked followed in 2010, by which time thalidomide was once again seen as a drug with much potential.

Professor Sheskin (center, in suit and tie) and his research team at the Jerusalem Hospital in 1980. (Photo: Public Domain/WikiCommons)
But in 2015, this trail of discovery reached a new peak: scientists have now discovered not only how to better use thalidomide, but they have struck upon ways the drug may be able to treat elusive diseases like cancer.
“What makes the story really bizarre, at least from a chemical biologist’s perspective, is that this is a compound that was made in the sense of ‘let’s put this in the body and maybe it’ll do something,’” says Dr. Joshiawa Paulk, a chemical biologist at the Dana Faber Cancer Institute. “But it ends up being this huge platform for designing, and it’s actually changing the way that we think about how to target things in cancer.”
A big reason why thalidomide works in cancer is because of its interaction with cereblon, a protein coded in the human genome. In the cells of complex organisms like humans, replication is vital, and happens on a constant basis. In the case of cancer, though, replication becomes a problem: cells mutate, grow out of control, and quickly form new, terrible cells. These cells behave oddly, and are desperately dependent on specific proteins that exist within them to live.
Normally, using specialized proteins, cereblon wanders around and flags unwanted material in your average, non-cancerous cell. Once flagged, material enters a sort of cellular garbage disposal system, and degrades. But when cereblon binds to thalidomide, which happens readily, it does something unexpected and amazing: it flags and destroys the exact proteins that cancer cells need to survive.

Artificial limbs were made in the 1960s for a child affected by thalidomide by the Department of Health and Social Security’s Limb Fitting Centre in Roehampton, London. (Photo: Science Museum London/WikiCommons CC BY-SA 2.0)
Paulk and his colleagues recently expanded on using the thalidomide-cereblon bond to target specific proteins, which kills their corresponding cancers. (Lung cancer cells depend on a different type of protein from blood cancer, for example). In their 2015 paper the scientists started with a molecule that is known to bind to a target protein. They then stitched the molecule to thalidomide–imagine a cellular Frankenstein’s monster–and the target was killed. Essentially, they have discovered how to throw any protein into the garbage using a cell’s own systems, by using molecules that Paulk says were once thought to be useless.
“Now we know that you can make things that bring the degradation machinery directly to what we want to destroy. It kind of changes the paradigm of what a small molecule can do,” says Paulk. “Probably in the next five to 10 years, it’s going to be really exciting for finding thalidomide-based novel therapeutics. It sort of changes the whole way that we think about drug discovery.”
Previously, most cancer treatments focused on killing the cancer cells through radiation treatments and tumor removal, not altering their behavior. The challenge for using biochemical therapies had been the complexity of finding out how to get the right molecules to target proteins into the cancer, and astonishly, thalidomide does that. Just this month, a new company called C-4 Therapeutics announced its intention to work with these new techniques, trademarked as Degronimid technology.

Grünenthal Headquarters in Stolberg. (Photo: Norbert Schnitzler/WikiCommons CC BY-SA 3.0)
Thalidomide isn’t perfect, clearly; there are reports that tolerance occurs over time, and with higher doses, patients experience neuropathy, a condition where extremities feel numb or painful. And despite strict drug regulations, in Brazil, where the medicine is used for leprosy, there was a spate of thalidomide-affected births in 2013.
In response to these problems, companies have made derivatives, one of which is marketed in the U.S. as Revlamid, so that some side effects of thalidomide are removed. It’s not yet clear if all side effects are preventable, though, and distributors continue to advise patients not to get pregnant or father a child while using it.
There’s undoubtedly still a lot of research to be done. In the meantime, it’s worth noting the bizarre paths that drug therapies can take. Off-label use and poor information caused thalidomide tragedies, but reexamining drugs and techniques can also have miraculous implications; those developed by Paulk and his colleagues do what once seemed impossible. “I’m sure many professors, PhD students, and post-docs have spent many, many years of their lives trying in vain to find the drug that would target [certain cancers], and failed,” says Paulk.
“But now, we can take those molecules, stitch on thalidomide, and that is going to be what directs the destruction. It’s not just blood cancer–we can now target any cancer that has a small molecule that binds to something [that kills it]. It’s really crazy.”














Follow us on Twitter to get the latest on the world's hidden wonders.
Like us on Facebook to get the latest on the world's hidden wonders.
Follow us on Twitter Like us on Facebook