The Hidden Memories of Plants
Inside a quiet revolution in the study of the world’s other great kingdom.
Monica Gagliano began to study plant behavior because she was tired of killing animals. Now an evolutionary ecologist at the University of Western Australia in Perth, when she was a student and postdoc, she had been offing her research subjects at the end of experiments, the standard protocol for many animals studies. If she was to work on plants, she could just sample a leaf or a piece of root. When she switched her professional allegiance to plants, though, she brought with her some ideas from the animal world and soon began exploring questions few plant specialists probe—the possibilities of plant behavior, learning, and memory.
“You start a project, and as you open up the box there are lots of other questions inside it, so then you follow the trail,” Gagliano says. “Sometimes if you track the trail, you end up in places like Pavlovian plants.”
In her first experiments with plant learning, Gagliano decided to test her new subjects the same way she would animals. She started with habituation, the simplest form of learning. If the plants encountered the same innocuous stimulus over and over again, would their response to it change?

At the center of the experiment was the plant Mimosa pudica, which has a dramatic response to unfamiliar mechanical stimuli: Its leaves fold closed, perhaps to scare away eager herbivores. Using a specially designed rail, Gagliano introduced her M. pudica to a new experience. She dropped them, as if they were on a thrill ride in an amusement park for plants. The mimosa plants reacted. Their leaves shut tight. But as Gagliano repeated the stimulus—seven sets of 60 drops each, all in one day—the plants’ response changed. Soon, when they were dropped, they didn’t react at all. It wasn’t that they were worn out: When she shook them, they still shut their leaves tight. It was as if they knew that being dropped was nothing to freak out about.
Three days later, Gagliano came back to the lab and tested the same plants again. Down they went, and … nothing. The plants were just as stoic as before.
This was a surprise. In studies of animals such as bees, a memory that sticks for 24 hours is considered long-term. Gagliano wasn’t expecting the plants to keep hold of the training days later. “Then I went back six days later, and did it again, thinking surely now they forgot,” she says. “Instead, they remembered, exactly as if they had just received the training.”
She waited a month and dropped them again. Their leaves stayed open. According to the rules that scientists routinely apply to animals, the mimosa plants had demonstrated that they could learn.
In the study of the plant kingdom, a slow revolution is underway. Scientists are beginning to understand that plants have abilities, previously unnoticed and unimagined, that we’ve only ever associated with animals. In their own ways, plants can see, smell, feel, hear, and know where they are in the world. One recent study found that clusters of cells in plant embryos act a lot like brain cells and help the embryo to decide when to start growing.
Of the possible plant talents that have gone under-recognized, memory is one of the most intriguing. Some plants live their whole lives in one season, while others grow for hundreds of years. Either way, it has not been obvious to us that any of them hold on to past events in ways that change how they react to new challenges. But biologists have shown that certain plants in certain situations can store information about their experiences and use that information to guide how they grow, develop, or behave. Functionally, at least, they appear to be creating memories. How, when, and why they form these memories might help scientists train plants to face the challenges—poor soil, drought, extreme heat—that are happening with increasing frequency and intensity. But first they have to understand: What does a plant remember? What is better to forget?

Scientists have shied away from studying what might be called plant cognition in part because of its association with pseudoscience, like the popular 1973 book The Secret Life of Plants. Certain types of plant memories were mixed up, too, with discredited theories of evolution. One of the most well-understood forms of plant memory, for example, is vernalization, in which plants retain an impression of a long period of cold, which helps them determine the right time to produce flowers. These plants grow tall through the fall, brace themselves during winter, and bloom in the longer days of spring—but only if they have a memory of having gone through that winter. This poetic idea is closely associated with Trofim Lysenko, one of the Soviet Union’s most infamous scientists.
Lysenko discovered early in his career that by chilling seeds he could turn winter varieties of grains, normally planted in the fall and harvested in the spring, into spring varieties, planted and harvested in the same growing season. He was, in essence, implanting a false memory of winter in plants that need a cold signal to grow. Despite this insight, Lysenko was not a very good scientist. But after he published early work on vernalization in the late 1920s, the Soviet government, looking for an agricultural panacea, inundated him with money and prestige. As Lysenko gained power, he made outrageous claims about his original idea. Vernalization, he said, could transform all kinds of plants, including potatoes and cotton, and boost the bounty of Soviet lands.

The evidence for these claims was scant, but that didn’t matter. By 1936, Lysenko led a major research institute and was a member of the Central Executive Committee, the nexus of Soviet power. With the help of a government-appointed philosopher, Lysenko developed a theory of his work that mixed Marxism with the discredited ideas of French naturalist Jean-Baptiste Lamarck. The offspring of vernalized plants, he argued, could inherit that acquired characteristic, so that by changing their environment he could create new breeds of staple crops in a fraction of the time of traditional breeding techniques—just as, by changing the environment of the working class, communism could create a new breed of men.
“All the claims were based on a principle of malleability, that genes were not all that important,” says Loren Graham, an emeritus historian at Harvard who has tracked Lysenko’s career. “Lysenko was a little unclear on the existence of genes.”
In practice, Lysenko’s theory fell apart. He couldn’t breed new varieties of grains that inherited memories of winter. He had promised fields fuller than ever before, but his ideas couldn’t save the country from famine in 1946 and ’47. And when geneticists challenged his ideas, Lysenko denounced them, which led to imprisonment and death for hundreds of scientists. He is often said to be responsible for creating a missing generation of Russian geneticists, who either gave up their work, left the country, or were punished for going against him. Without them, Lysenko never could see where he was right (plants could form these memories of winter) and where he had gone wrong (this type of memory, at least, cannot be transmitted across generations). It took a generation of scientists, working in the West, to uncover the true secrets of the phenomenon Lysenko claimed as his own but never truly understood.
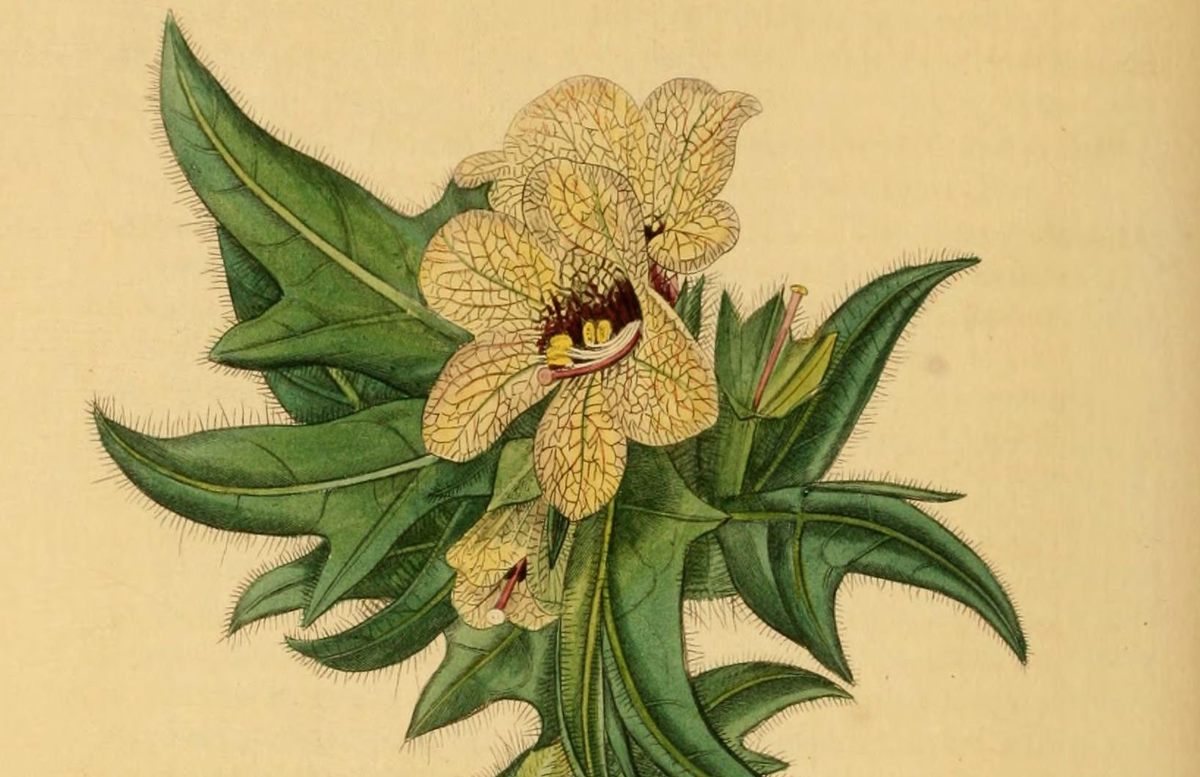
Even as Lysenko was making his grandiose claims, scientists on the other side of the Iron Curtain were trying to understand how vernalization works. Some of the most important investigations to examine this mystery took place in Tübingen, Germany, in the lab of Georg Melchers and Anton Lang. Melchers was a leading biologist of plant development, and Lang was a stateless, refugee Russian biologist. Together they studied vernalization in search of the biochemical secret of flowering, a hypothetical plant hormone scientists called “florigen.”
One of their study subjects was a nightshade called henbane, Hyoscyamus niger. Some plants flower after reaching a certain point in their development, like teenagers who hit puberty and start to parade their newfound sexuality immediately, regardless of the consequences. Other plants, though, behave more like teenagers who wait for summer break to go crazy: They only flower when they receive cues from their environment that it’s the ideal time to do so. Henbane is one of the latter and requires both a period of cold and the right light to bloom. Rather than growing and dying in one season, as annual plants do, certain varieties of henbane are biennials, with a life cycle that spans two growing seasons. In their first spring and summer, these plants grow as much as they can, but hold back from flowering. Only in the following spring do they burst into bloom—creamy white flowers smudged in their centers with red-wine purple that runs through the veins on their petals. For a biennial, these dual requirements make sense: They prevent the plant from flowering in the fall, when the light is right but the cold days of winter would doom their flowers.

While trying to understand how vernalization and day length work in concert to make henbane flower, Melchers and Lang probed the limits of the plant’s memory of winter. In one experiment, they vernalized the plants by chilling them in a fridge and then tried to reverse the process by blasting them with warmth. The plants, they found, formed lasting impressions of the cold relatively quickly. After a day or two of chilling, the scientists could still “de-vernalize” the plants, but after four days, that possibility had vanished—the plants remained vernalized. In practice, this means that a warm spell in February won’t trick henbane into forgetting the cold weeks they have experienced. In another experiment, they withheld the ideal day length. The vernalized plants continued to grow but never flowered. Even after 10 months, when they were exposed to the day length that told them it was the right time, they would still bloom. They had remembered that experience of cold for close to a year.
Melchers and Lang didn’t describe vernalization as a “plant memory,” but today it’s one of the most studied examples. Their experiments showed that plants could hold on to their pasts, for much longer than a person might expect, like undercover agents, fully trained but awaiting the signal to act.

When most people look at a plant, it’s hard to imagine that it’s waiting for anything. Plants don’t seem to have long-term plans. If they lack water, they droop. If it rains, they perk up. If they sense sunlight, they grow toward it. To our human way of thinking, it doesn’t seem like plants are doing very much at all. But we don’t recognize memories in people or dogs just by looking at them, but rather by their behavior. The dog comes when called by name; the person smiles in recognition. For the mimosa or henbane plants, something from the past changed how they reacted in the future—even if we don’t notice or understand why.
Scientists first started talking about “plant memory” explicitly in the 1980s. A team in France, for example, happened upon a type of memory in which a plant recalled a history of damage to a leaf on one side of its stem and therefore dedicated its energy to growing in the other direction. Since then, scientists have found that certain plants can remember experiences of drought and dehydration, cold and heat, excess light, acidic soil, exposure to short-wave radiation, and a simulation of insects eating their leaves. Faced with the same stress again, the plants modify their responses. They might retain more water, become more sensitive to light, or improve their tolerance to salt or cold. In some cases, these memories are even passed down to the next generation, as Lysenko thought they could be, though in an entirely different way than he imagined. We now know that plants are capable of much more than they’re given credit for. They can “hear” vibrations, which might help them recognize insect attacks. They share information by broadcasting chemicals through the air or from their roots. In the study of the memories they form, the next step has been to understand how they do it.

In Melchers and Lang’s time, hormones were the leading edge of plant science. The technique for discovering new hormones was elegantly brutish: Scientists ground up leaves before extracting and isolating the small molecules they released. They then sprayed the hormones back onto plants to see what happened. Gibberellin, for instance, stimulates growth. Today, it’s sprayed on grapes to make the fruit fatter and less tightly clumped. “A great deal of plant physiology was looking for these types of signals,” says Richard Amasino, a professor of biochemistry at University of Wisconsin-Madison. “But the signals in flowering had not been found, despite a lot of grinding up plants.”
By the 1970s and early ’80s, plant scientists still hadn’t found the biochemical secret to flowering. “When I began in science, this was a big mystery,” says Amasino. To understand it and begin to unlock plant memory, scientists needed the insights of molecular genetics and, in particular, epigenetics, the mechanisms that switch particular genes on and off.
In recent years, scientists have realized that the genome alone doesn’t determine an organism’s fate. There’s a whole world of epigenetic activity around DNA that impacts which stretches of code get expressed, or translated into action. Florigen turned out to be a tiny protein, too small for the techniques of Lang’s generation to identify. Even if they had found it, they would have been missing a key to the mystery of what makes biennials flower. Amasino’s generation, on the other hand, finally found the right level of activity—the epigenetic level—to see this process in action.
For example, the mechanism that controls vernalization and flowering in Arabidopsis thaliana, or thale cress, a plant often used as a model in laboratories, is like a Rube Goldberg device of proteins and gene expression. The plant has a set of genes that create the proteins that cause flowers to form. Before vernalization, the cells are full of a second protein, named FLC, that represses those key, flower-promoting genes. But when the plant is exposed to cold, its cells slow the production of FLC until it stops, and the balance of protein power then changes. The cells start producing more and more flower-promoting proteins, until the plant is ready to burst into bloom. In this case, a simple way to think about this epigenetic action is as a switch. The cold acts as a signal to the cell to switch the way its genes are expressed, from “don’t flower” to “flower, flower, flower.” And even when the cold signal is gone, the switch stays flipped. So, when days lengthen, the plants know it’s the right time to bloom.
“Even when it’s spring and summer,” explains Amasino, “whatever the cold did remains as a memory.”

Are plants secretly soaking up memories, flicking their epigenetic switches on and off in response to every significant stimulus they receive? It seems unlikely. Last year, a group of plant scientists based in Australia argued in the journal Science Advances that, for plants, forgetting (or not forming memories at all) may be a more powerful tool for survival than memory, and that “memory, in particular epigenetic memory, is likely a relatively rare event.”
Peter Crisp, the lead author of the paper, now at University of Minnesota, makes it his job to stress plants out. He and his colleagues might stop watering plants and let them dry out before hydrating the thirsty plants and watching how they recover. It’s been established that in certain plants, epigenetic memories of drought, along with other stressors such as low light and herbivory, can even make the leap across generations. So Crisp and his colleagues might do this for multiple generations (it gets interesting after three) before testing if the plants remember the ordeal they’ve been through and become more tolerant of drought. “We don’t really see that,” says Crisp.

Plants, he points out, have incredible abilities to rebound from stressful conditions. In a paper published this summer, for instance, Crisp and his colleagues found that plants subjected to light stress rebounded rapidly—just think how, with the right care, a neglected houseplant can bounce back from a wilted, brown mess. Scientists have now reported plenty of examples of plant memory formation, but naturally they are less likely to publish results of experiments where plants could potentially form memories but don’t. One of the biggest challenges of the field of study is even identifying whether a plant has formed a memory or not.
When Crisp and his colleagues design lab studies, they have to control for any number of confounding factors to determine if any memories they observe are the result of the experimental stress. “It’s not as though the plant experiences something and says, ‘Oh, I remember this,’” says Steven Eichten, Crisp’s coauthor, from the Australian National University. “It happens to have this chemical marker, a change at a molecular level.” Identifying that change and attributing it to the experimental stress can be difficult. Even when scientists know a memory can form in one plant, they may not necessarily recognize it in another. The memory mechanism involving FLC that Amasino worked on, for instance, only works in thale cress. Beet plants and wheat plants have their own molecular mechanisms of vernalization, which serve the same function but evolved independently. Identifying a true memory out in the field is significantly harder.
In their experiments, however, Crisp and Eichten don’t observe many plant memories being formed. What if, they ask, plant memory is rare simply because it’s better for plants to forget? “Having a memory, keeping track molecularly of signals that you’ve received in the past from your environment, does have a cost,” says Eichten. “Since we don’t see memories all that often … maybe plants don’t want to remember things all the time. Maybe it’s better to put their energies elsewhere.” Even when memories do form, they can fade. Another research group has shown, for example, that a plant might form an epigenetic memory of salt stress and pass it along across generations, but that if the stress fades, so does the memory. A plant that remembers too much might sacrifice healthy growth to be constantly on guard against drought, flood, salt, insects. Better, perhaps, to let those negative experiences go, instead of always preparing for the worst.

It’s inevitable that we try to understand plant memory and cognition through our own experience of the world. To an extent, even using the word “memory” is an evocative anthropic shorthand for what is actually going on in these plants. “We use the term ‘plant memory,’ but you could find other ways to try to describe it,” says Eichten. But “semi-heritable chromatin factors” doesn’t quite have the same legibility. “Sometimes I have to try to explain my work to my mom, and you say, ‘Well, maybe it’s like a memory … ’ Even if you think about human memories, it’s still kind of an abstract thing, right? You can think about neural connections, but often in common dialogue, when you think of memory, you know what a memory is. At that level, maybe you don’t care about where it’s coming from or what specific neurons are tied to it.”
That’s closer to the position from which Gagliano, the ecologist, approaches plant memory. Unlike the molecular geneticists, she’s less interested in the specific mechanisms of memory formation than she is in the process of learning itself. “Of course plants can remember,” she says. “I know that behaviorally a plant will exhibit a change in behavior that is predictable—if condition A is met, then the plant should be able to do X. So by being able to do X, it means the plant has to remember what happened before, otherwise he wouldn’t be able to do X.”
The leaf-closing M. pudica isn’t the only plant that Gagliano can teach new tricks. In another experiment, she grew garden pea plants in a Y-shaped maze and tested whether they could learn to associate different cues, wind and light. Plants gravitate toward light, and in the experiment, Gagliano added an additional cue, airflow produced by a fan. For some of the plants, light and air flowed from the same side of the Y-shape. For others, light and air came from different directions.

“With the peas, I turned the dial up,” she says. “Not only did the pea need to learn something, but he learned something that meant nothing, that was totally irrelevant. Mimosa had to follow just one experience, the drop, ‘What does this mean?’ While the pea had to follow two events occurring”—the fan and the light.
After training the plants, Gagliano withheld the light. When she next turned on the fans, she had switched them to the opposite branch of the Y shape. She wanted to see if the plants had learned to associate airflow with light, or its absence, strongly enough to react to the breeze, even if it was coming from a different direction, with no light as a signal. It worked. The plants that had been trained to associate the two stimuli grew toward the fan; the plants that had been taught to separate them grew away from the airflow.
“In that context, memory is actually not the interesting bit—of course you have memory, otherwise you wouldn’t be able to do the trick,” she says. “Memory is part of the learning process. But—who is doing the learning? What is actually happening? Who is it that is actually making the association between fan and light?”
It’s telling that Gagliano uses the word “who,” which many people would be unlikely to apply to plants. Even though they’re alive, we tend to think of plants as objects rather than dynamic, breathing, growing beings. We see them as mechanistic things that react to simple stimuli. But to some extent, that’s true of every type of life on Earth. Everything that lives is a bundle of chemicals and electrical signals in dialogue with the environment in which it exists. A memory, such as of the heat of summer on last year’s beach vacation, is a biochemical marker registered from a set of external inputs. A plant’s epigenetic memory, of the cold of winter months, on a fundamental level, is not so different.






















Follow us on Twitter to get the latest on the world's hidden wonders.
Like us on Facebook to get the latest on the world's hidden wonders.
Follow us on Twitter Like us on Facebook